Chemists Use MASCoT to Create New Metalloproteins
Simple changes to amino acids lead to formation of complex supramolecular structures
March 5, 2019 | By Cynthia Dillon
An old design adage of the Navy’s—KISS (keep it simple, stupid)—could sum up a new approach to engineering metalloproteins. Accounting for nearly half of all proteins in nature, these proteins with metal ions serve as catalysts for important biological process like photosynthesis, respiration and water oxidation by providing bonding sites for molecules. Designing new functional proteins (e.g., enzymes), however, from scratch can be extremely challenging. But researchers from UC San Diego and UC Irvine were able to devise an approach to meet the challenge with a new strategy coined “Metal Active Sites through Covalent Tethering” (MASCoT). Details are outlined in a paper recently published in Nature Chemistry.
“With only four mutations, an arbitrary electron transfer protein is converted into a versatile metalloprotein complex,” explained Akif Tezcan, professor in the Department of Chemistry & Biochemistry at UC San Diego.
International scientists have noted the research findings. In a recent Chemistry World article, Gerard Roelfes from the University of Groningen in the Netherlands, described the strategy as unique.
“The beauty of the present approach is its simplicity: it just requires introducing a cysteine at a certain position and then dimerising the protein and introducing a few metal binding residues,” stated Roelfes. “If they can actually create enzymes using this approach, then it has a bright future.”
According to Tezcan, it can be particularly difficult to create what he calls a “well-defined 3D environment” such as a binding pocket that can interact with a particular molecule or substrate.
“Most protein and metalloprotein engineering efforts depend on the repurposing of pre-existing protein folds with well-defined active sites in their interiors,” he explained. “In our lab, we have taken an alternative strategy in which we use well-folded proteins as building blocks for forming new supramolecular complexes, and the newly created interfaces in these complexes are then used to build new functional sites.”
The approach works by joining smaller protein units together to make a large three-dimensional structure with a metal coordination site, which Tezcan and his research colleagues demonstrate can be achieved with simple modifications to some of the amino acids. By using MASCoT, the team was able to create metal coordination sites capable of binding transition metal ions such as manganese, copper, nickel, cobalt and zinc to make a variety of metalloproteins. They also showed they could make an iron metalloprotein that could bind to nitric oxide.
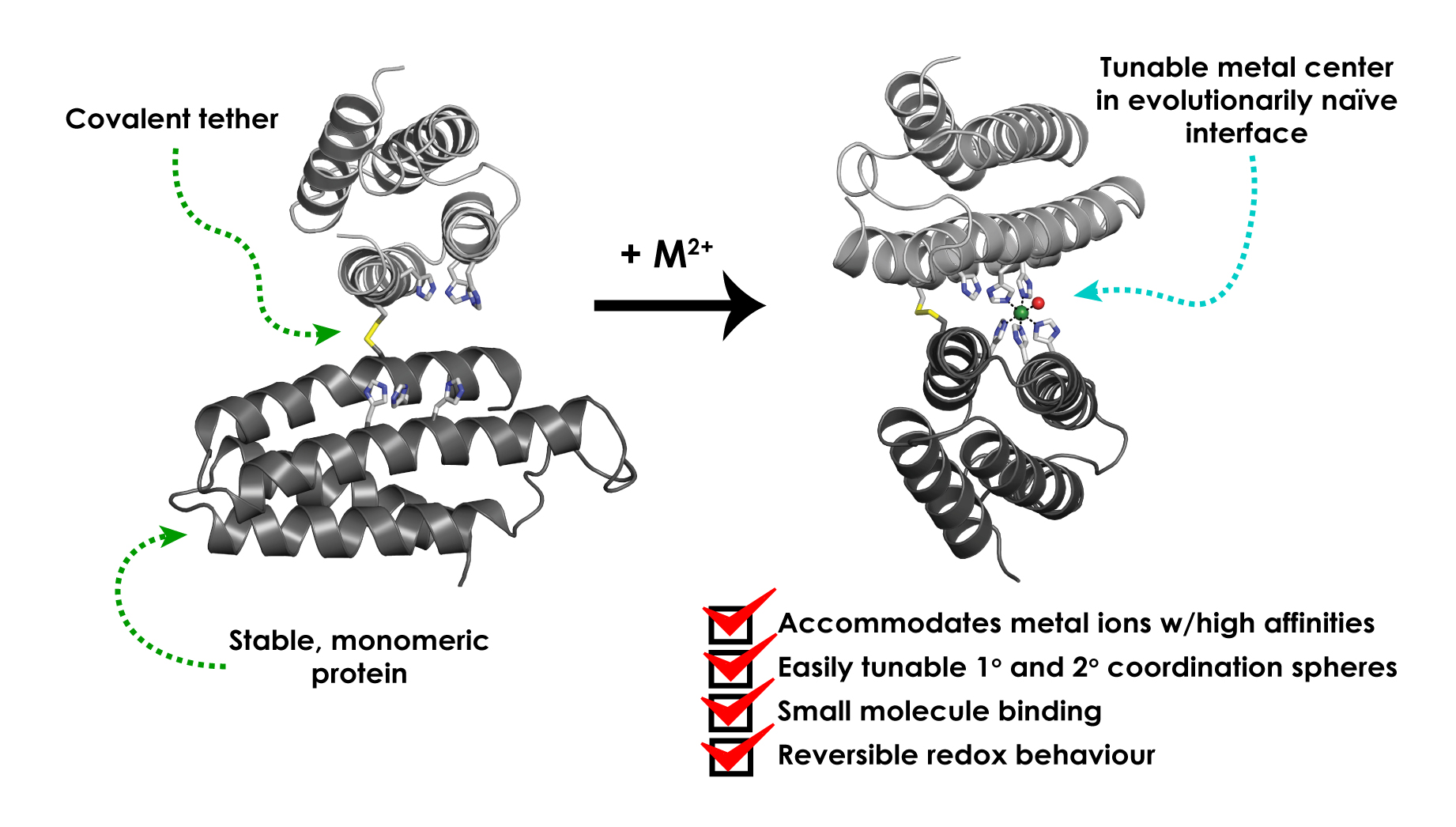
Graphical representation of implementation of MASCoT. Figure courtesy of © Jonathan Rittle et al/Springer Nature Limited 2019
A few years ago, Tezcan’s group developed an approach which used metal ions to link different protein modules together into complex three-dimensional structures, which they subsequently used to build a functional metalloenzyme. In their most recent work they streamlined this approach, showing it is possible to create new protein–protein interfaces by mutating a single cysteine residue in the protein cytochrome cb562, which allows it to join to another cytochrome cb562 via a disulfide linkage.
“I think the power of this strategy lies in how easy it is to implement and how efficient it is,” said Tezcan, adding that, in principle, the approach can be easily carried out with many other protein building blocks. It can also be complemented by computational protein design to optimize desired structural and functional properties.
In the short term, he said that the research team wants to build metalloenzymes with catalytic activities including redox chemistry and small molecule activation. Long term, the researchers want to use MASCoT to design metalloproteins that are active in living systems and able to utilize non-biological metal ions.
This research was supported by the National Science Foundation (grant no. CHE1607145 to F.A.T.) and a postdoctoral fellowship from the National Institute of General Medical Sciences of the National Institute of Health (grant no. F32GM120981). Portions of the research were carried out at the Stanford Synchrotron Radiation Lightsource at the Stanford Linear Accelerator Center and the Advanced Light Source at the Lawrence Berkeley National Laboratory, which are supported by the DOE, Office of Science, and Office of Basic Energy Sciences (contracts DE-AC02-76SF00515 and DE-AC02-05CH11231, respectively). The researchers also thank C. Moore and M. Gembicky for assistance with XRD experiments.

