New member of protein family reveals an ancient role in iron metabolism
May 29, 2012
Discovery of a protein that is involved in many metabolic processes in a plant offers insight into the ancient functions of a little-understood but important player in regulating physiology.
Scientists at the University of California, San Diego, the University of North Texas, and Hebrew University of Jerusalem, have identified a plant version of NEET, a class of proteins distinguished by a unique fold or pocket that holds a custer of iron and sulfur ions, they report in the scientific journal Plant Cell.
“We’re gaining insight into how this protein may be involved in metabolism, particularly the management of iron levels within cells,” said Patricia Jennings, professor of chemistry and biochemistry and UC San Diego.
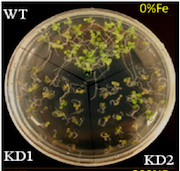 The team found the new protein in the mustard plant, Arabidopsis thaliana, a species commonly used for genetic studies, and called it At-NEET.
The team found the new protein in the mustard plant, Arabidopsis thaliana, a species commonly used for genetic studies, and called it At-NEET.
They located At-NEET in the plants’ chloroplasts and mitochondria, both structures within cells that harbor many proteins that need iron to function properly. To build those proteins, cells must somehow collect iron and attach it to the proteins. NEETs seem to serve that transfer function.
The team also directly showed, biochemically, that At-NEET can donate clusters of iron and sulfur to other proteins and structures, including mammalian mitochondria, depending on the tightness of that special pocket, suggesting that the iron-transfer function of this class of proteins is ancient.
“What we are seeing with the NEET proteins is that the iron-sulfur centers dictate the protein’s function in cells,” said John Zuris, a graduate student win Jennings’s research group and one of the authors of the report. “Any changes we make to the components that hold the iron in place dramatically alter function.”
The health of whole plants also depends on sufficient levels of At-NEET. Wan and shriveled, mustard seedlings with low levels of At-NEET fail to thrive. They accumulate iron, but fail to incorporate it into their chloroplasts or into the enzyme catalase, which is critical for mopping up damaging reactive oxygen, suggesting that At-NEET regulates where iron ends up within the cells.
Mustards with litte At-NEET grow slowly in iron-poor medium on which normal mustards do just fine, suggesting that they can’t gather iron from the environment or can’t move it to where it needs to be in the plant. Oddly, that may protect the plants in some environments; they survive iron-rich medium that proves toxic to normal seedlings.
Humans and other mammals have a several NEET proteins, including one called mito-NEET. Diseases such as diabetes and have increasingly been associated with mitochondrial dysfunction. A better understanding of this class of proteins could lead to drugs that act in new ways. In fact, mitoNEET is a target of the diabetes drug pioglitazone.
Mammals’ multiple versions of NEET hamper studies of their functions because they compensate for one another; knocking down one NEET protein boosts the activity of the others, obscuring some of the effects. Mustards, with just one version of NEET, may offer a simpler model for understanding the proteins’ functions.
At-NEET may also be valuable as a way to boost the resilience of plants to feed the growing human population. “Plant stress is an important world health issue,” Jennings said. “You need to be able to grow on deficient soils. You need drought tolerance and heat tolerance. At-NEET is one of the regulators of the stress reponse.”
Additional authors of the paper include members of research groups led by Jennings at UC San Diego, Ron Mittler at the University of North Texas, and lead author Rachel Neuchushtai at Hebrew University of Jerusalem, along with additional colleagues at Hebrew University.
